mRNA therapeutics and vaccines
Why mRNA represents a disruptive new drug class
Messenger RNA, or mRNA, represents one of the first molecules of life and is found in all living cells. It is composed of four different building blocks called nucleotides. An mRNA molecule consists of hundreds or thousands of nucleotides that are linked in a unique order to convey the necessary information to manufacture the corresponding protein in cells. In a therapeutic setting, mRNA can be used as a medium to encode the proteins of choice.
At a glance: mRNA as a versatile drug class
- Natural molecule found universally in cells, with well-researched properties
- Suitable to encode for antibodies, antigens, cytokines, and all other proteins
- Transient, with adaptable activity and half-life. Avoids genomic integration problems that sometimes occur in gene therapy, potentially leading to a better safety profile
- Can be designed and optimized pharmacologically and immunologically, making it suitable for a broad range of applications
- Fast manufacturability allowing for flexible therapeutics
Leveraging mRNA to develop meaningful therapeutics and vaccines
We have developed a range of mRNA platforms to bring a new class of therapeutics and vaccines to patients and individuals who need them most. These scientific discoveries and advancements have enabled us to pioneer the development of the first mRNA-based vaccine and we are expanding our expertise to prevent various infectious diseases with our mRNA vaccine platform.
Our cancer vaccine platforms FixVac and iNeST aim to revolutionize cancer treatment as we know it providing cancer patients with a more tailored treatment approach specific to a certain tumor indication (FixVac) or even to their specific tumor (iNeST). In addition, we are leveraging mRNA to deliver the building instructions for a targeted antibody or cytokine or immunomodulating protein directly to the patient with our RiboMab, RiboCytokine and Intratumoral Immunotherapy platforms helping the body to produce its own therapeutic directly in the tumor.
Read more about our mRNA platforms here
How BioNTech’s fundamental and translational cancer research paved the way for the first commercially available mRNA vaccine
To develop an mRNA-based cancer vaccine, BioNTech co-founders Ugur Sahin and Özlem Türeci faced three major challenges that were previously considered insurmountable:
- The vaccine had to be capable of inducing billions of immune cells because small tumors consist of billions of cancer cells.
- The tumor characteristics that need to be recognized by the immune system are unique to each patient. Sahin and Türeci recognized that tailoring vaccines to each patient’s antigen profile would require an individualized treatment.
- An individualized vaccine has to be manufactured very rapidly to halt the spread of cancer in patients.
Over the next two decades, Sahin, Türeci, and their team achieved a series of scientific and technological breakthroughs to unlock the full potential of this molecule for cancer vaccination.
- They worked on discovering and engineering structural components of mRNA (cap, the poly-A tail and its untranslated regions), which increased the intracellular stability of mRNA and its translation efficiency. By combining the improved elements, protein expression could be increased by more than 1,000-fold.
- They improved the efficacy of the vaccine when they discovered the mechanism of selective mRNA uptake into dendritic cells (DCs), specifically the DCs located in lymphoid tissues. These DCs, the "high-performance trainers" of the immune system, boost immune response (CD8 and CD4 T cells) and therefore the potential effectiveness of the treatment significantly.
- The team also identified a method to improve antigen presentation by dendritic cells for stronger T cell responses by coupling the antigens with a Major Histocompatibility Complex I (MHC-I) trafficking signal.
- To realize the vision of individualized vaccine technology, the team developed a breakthrough approach that can be applied to different types of cancer and made available for clinical trials in just a few weeks.
The lessons of this process contributed to the rapid availability of the COVID-19 mRNA vaccine.
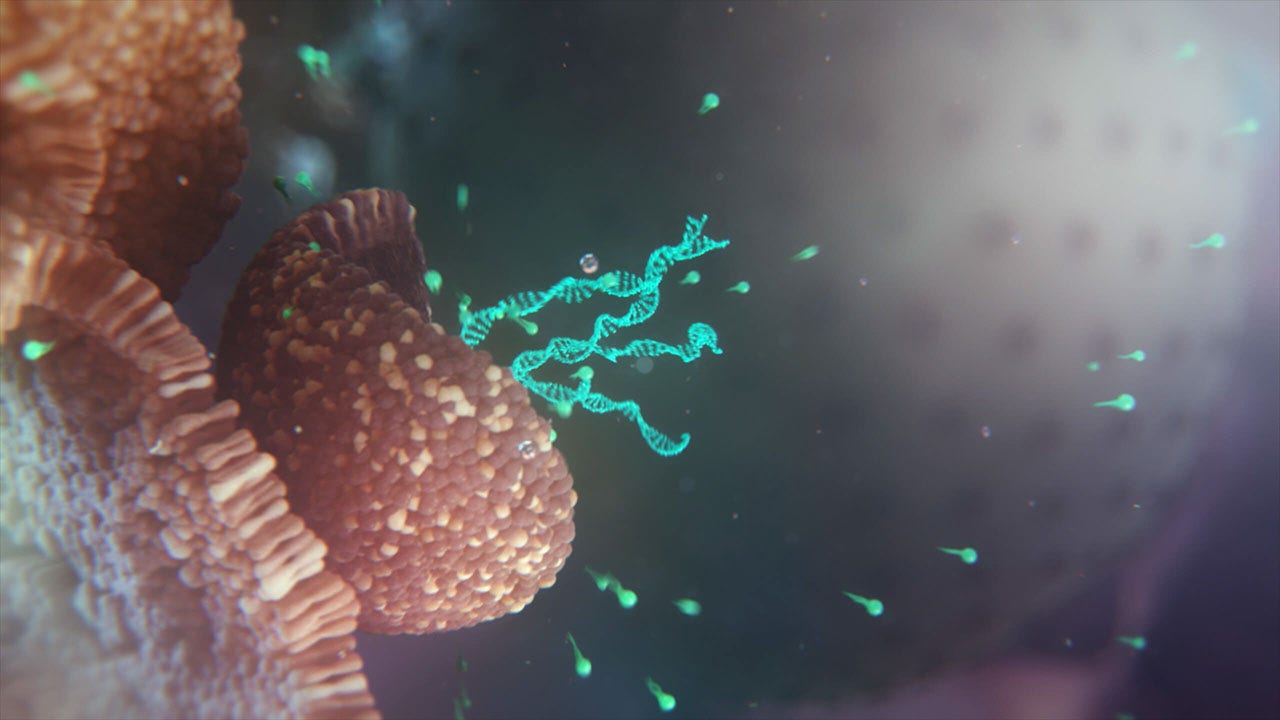
Structural improvements to make mRNA a powerful drug class
All our mRNAs contain optimized structural components that we believe are critical for successful development:

5’ cap
Incorporating a unique cap analogue into the mRNA drives strong translational performance by stabilizing the mRNA molecule and directing the immune response.
3’ untranslated region
The composition and structure of the 3’ untranslated regions of the mRNA molecule help determine the intracellular stability of mRNA.

Poly(A) tail
We have conducted extensive research on the structure of the poly(A) tail and the translational performance of mRNA, and customized our template design accordingly.
Pioneering a new path with solution-driven approaches
Over several years of research, Ugur Sahin, Özlem Türeci and Katalin Karikó solved the basic problems of RNA-based therapeutics and vaccines, using different, independent approaches. The main issues were the inflammation caused by the immune response to enormous doses as well as the low and short-lived protein production.
While focusing on mRNA therapies for the treatment of strokes and cerebral diseases, Karikó discovered that modifying the nucleotide uridine in mRNA with a naturally occurring alternative, such as methyl-pseudo-uridine, the mRNA was not detected by the immune system as a foreign molecule that avoided a strong inflammatory response. This allowed mRNA to be administered in higher doses.
Sahin und Türeci chose a different approach to address the problem of low immunogenicity: they achieved a series of scientific and technological breakthroughs to unlock the full potential of this molecule by discovering and developing a design for each of the structural components of mRNA (cap, the poly-A tail and its untranslated regions). This approach, in turn, increased the intracellular stability of synthetic mRNA as well as its translation especially in immune cells leading to lower doses needed to induce a significant immune response. mRNA thus became potent enough to be used as a drug.
Katalin Karikó was a part of the BioNTech family since 2013, since 2022 she works as an external consultant for the company. The three scientists published a comprehensive overview of the possibilities of the mRNA platform, in which they played a major role, in Nature Reviews Drug Discovery in 2014.
Our mRNA formats
The nucleotide sequence of mRNA determines the amino acid sequence of the protein. In addition, the nature of nucleosides used for production of mRNA drugs can also influence how the immune system recognizes the molecule. The presence of naturally occurring uridine (U) in our optimized mRNA makes it immunogenic by activating immune sensors. We have optimized our uRNA for immunogenicity (augmented antigen presentation on MHC I and MHC II) and pharmacological activity (enhanced stability and translational efficiency). By acting as an immunotherapy adjuvant, immunogenicity of the uRNA is beneficial when used for immunotherapy applications – resulting in potentially more potent therapeutics for iNeST and FixVac which are currently being evaluated in the clinical setting.
We must avoid immunogenic reaction against mRNA drugs in applications where therapeutic proteins are produced, such as in our RiboMab and RiboCytokine platforms. BioNTech has significant expertise in incorporating naturally occurring modified nucleosides into our therapeutic mRNAs. We have demonstrated that incorporating a variety of modified nucleosides in the manufactured mRNA suppresses its intrinsic immune activation, while leading to superior protein production over time. Deimmunizing mRNA by incorporating modified nucleosides helps to avoid production of anti-drug antibodies and broadens the therapeutic application of these types of mRNA drugs.
Our self-amplifying mRNA (saRNA) drugs use the concept of viral replication, while not being an infectious, disease-causing agent itself. saRNA resembles the process of conventional mRNA encoding the protein of interest, but also encodes a polymerase – called replicase – that multiplies part of the mRNA within the target cell. During self-amplification inside the cell, a double-stranded RNA intermediate is generated, which is recognized by intracellular immune sensors. This makes saRNA a very potent activator of the immune system and therefore an excellent category of immunotherapy. We believe that saRNA ensures high levels of sustained antigen production with a small amount of initial mRNA input, which could make this mRNA class ideal for prophylactic vaccination with potential application for infectious diseases.
This technology is an advancement of the saRNA platform. By separating the target mRNA to be amplified from the replicase encoding mRNA, we can develop more applications. This makes the development of therapeutic mRNAs even more flexible, as the replicase can amplify mRNA encoding of several different proteins. This allows us to produce the replicase in advance for use with different vaccine candidates. Our trans-amplifying mRNA is a proprietary mRNA format that we believe is well-suited for prophylactic vaccines to prevent infectious diseases.
Delivery Formulations
Our deep and broad expertise in the targeted delivery of mRNA therapeutics
BioNTech has made great progress in developing cancer vaccine candidates as well as additional vaccine candidates against infectious diseases. The development of suitable lipid nanoparticle formulations for the well-protected and targeted delivery of mRNA is a key achievement made by our scientists. Ugur Sahin and Özlem Türeci discovered the mechanism of selective mRNA uptake into dendritic cells and, with their team, developed a lipid nanoparticle formulation that exploited this mechanism to target RNA into these cells.
We employ multiple mRNA delivery formulations, each designed for different functions and optimized for therapeutic product needs based on the intended application and route of delivery:
Lipoplex
Our lipoplex formulation, or LPX, embeds the mRNA between a lipid bilayer, which is used for our FixVac and iNeST platforms. We use a proprietary size- and charge-based non-viral mRNA lipoplex that was developed to deliver mRNA to dendritic cells in lymphoid compartments such as the spleen and the lymph nodes for optimal antigen presentation and immune response activation.
Lipid Nanoparticles (LNPs)
For other applications, we encapsulate our mRNA in lipid nanoparticles, or LNPs. These formulations are suitable for our RiboMab and RiboCytokine platforms. LNPs have been used also for our COVID-19 vaccine.
Polyplexes
Our portfolio also comprises polyplexes, used in some of our discovery programs, in which the mRNA is bound to a polymer and then forms nanoparticles.